Harm reduction has been defined by SAMHSA as: “…an approach that emphasizes engaging directly with people who use drugs to prevent overdose and infectious disease transmission, improve the physical, mental, and social well-being of those served, and offer low-threshold options for accessing substance use disorder treatment and other health care services.”
It’s a definition that covers a lot of territory. Examples of harm reduction programs include needle exchange, naloxone distribution, and safe injection centers. Because such approaches may not aim to get users to stop using drugs – in some ways, it’s an acknowledgment that they probably won’t stop, at least not yet, regardless of our efforts – harm reduction programs can face opposition. Still, in view of the rising number of deaths by OD, harm reduction approaches are being adopted by a number of states and communities.
I thought I’d mention three important examples. First, from a recent piece in The New York Times:
Fentanyl Test Strips Highlight Rift in Nation’s Struggle to Combat Drug Deaths
The aim is to let the user know that super-potent fentanyl may also be present in the opioid or stimulant or sedative they are about to ingest. That’s a genuine risk now because fentanyl is cheap, powerful, and widely available as an additive. Of course, a user who knows fentanyl is present may still decide to go ahead and shoot up. Public health officials have to hope that the greater number will be deterred and go looking elsewhere to get high.
As for how much impact testing strips would have on overdose rates, I wouldn’t predict. Street addicts are not the most compliant folks, and are known to be prone to risky behaviors even when aware of the consequences. We’ll have to wait and see.
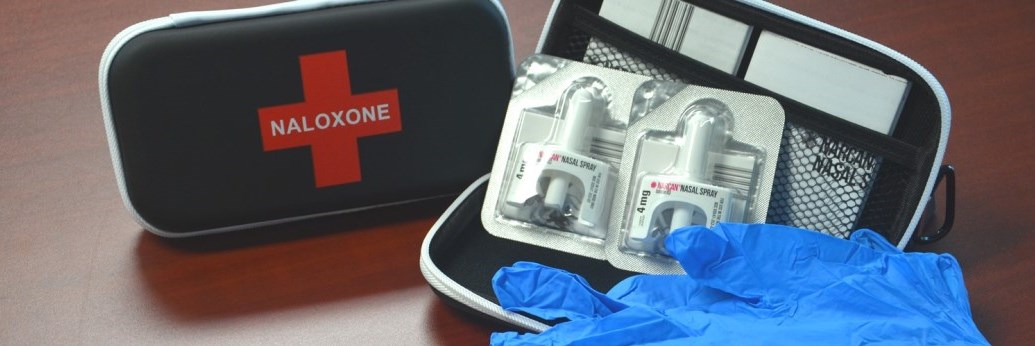
Same goes for this next strategy: making naloxone, the opioid antagonist used to revive overdose victims, available as an over-the-counter product. That story:
FDA advisers recommend approval of over-the-counter naloxone to fight opioid overdose
This is only in the nasal spray form. That is effective in reversing some but not all ODs. If approved by the FDA, naloxone spray will be available in retail stores and pharmacies, even in vending machines. No prescription needed.
How much impact will that have on OD rates? Probably more than the test strips alone. The hope is that many users will take advantage of both.
The third and final proposal – one advocates have long pursued – involves removing a requirement that physicians who want to prescribe buprenorphine (Suboxone, etc) to patients who have OUDs need to first obtain a waiver, including education and training about the drug and its use.
The X-waiver for buprenorphine prescribing is gone. It’s time to spread the word
I confess that a bit of medical education always seemed to me like a good idea. I’ve met a lot of doctors, after all. Nonetheless, advocates insist the waiver requirement has been a major reason that so few MD’s have enrolled as prescribers.
Once again, I won’t try to predict how much impact this or the other changes will have on addict behavior or on the course of the epidemic itself.
No need for guesswork. The answers will be self-evident, before too much time has passed.